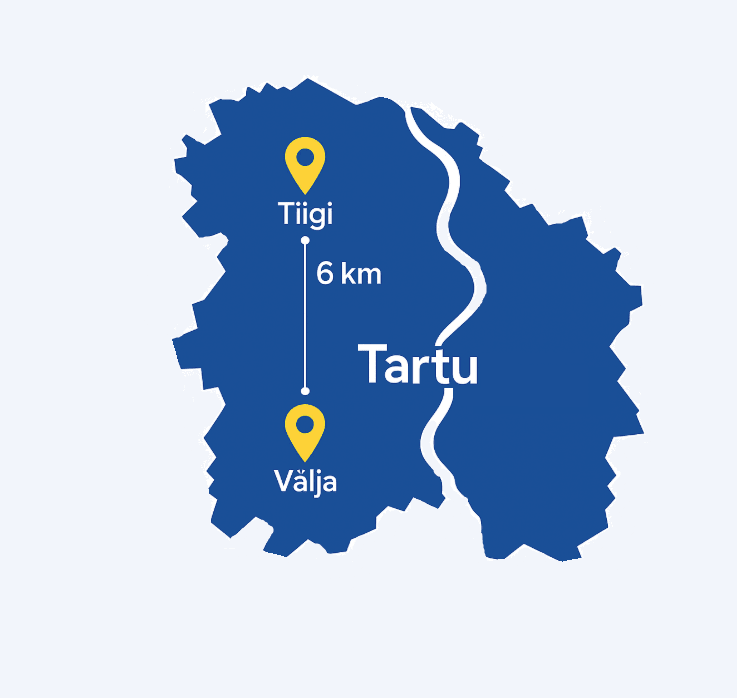
Our Tiigi facility, located at Tartu Biotechnology Park (Tiigi tn 61b, 50410 Tartu, Estonia), serves as TBD Pharmatech’s central hub for research and development, analytical quality control, and small-scale GMP manufacturing activities. The facility integrates approximately 1000 m² of GMP-certified laboratories and production areas, accommodating early-phase API development, scale-up processes, and clinical batch manufacturing. Alongside corporate offices and administrative functions, Tiigi features eight dedicated cGMP-qualified production rooms capable of batch sizes ranging from milligram to 150 liters.
Our RnD and analytics laboratories provide comprehensive capabilities, including model and high-pressure reactors for process optimization, sophisticated preparative chromatography techniques (Flash chromatography, Prep-HPLC), vacuum-based systems for evaporation and drying, and extensive analytical instrumentation such as HPLC systems (with DAD and CAD detection), HPLC-MS, GC (including GC-HS), Karl Fischer titration, polarimetry, viscosimetry, and automatic titration methods.