Your EU-based small molecule contract development and manufacturing (CDMO) partner for the long run
We are a CDMO with 17 years of experience in the market. We combine EU GMP-compliant production with strong R&D in both synthesis and analytical methods as well as extensive experience in the process validation, fast and open communication on top of that — something that makes TBD the perfect partner to develop and execute your ideas if you are a pharmaceutical or a biotech company.
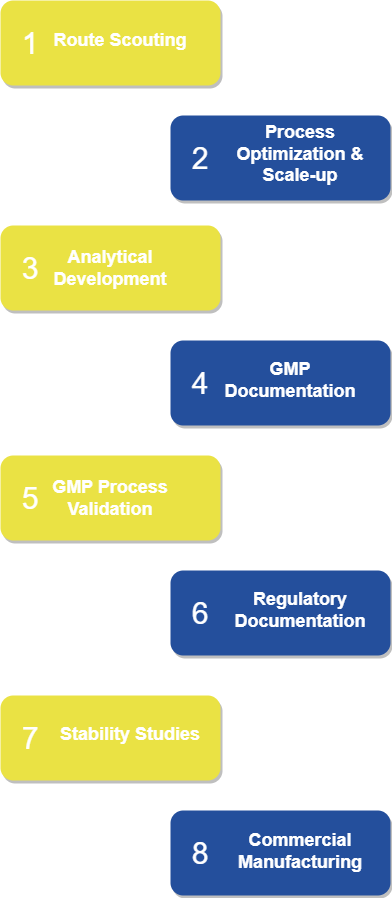
Offering full range of contract development and manufacturing services in the field of human and veterinary pharma from route scouting to CMC documentation, TBD excels in development and manufacturing of API`s for generics and Phase 1-2 trial API`s for innovative medicines.